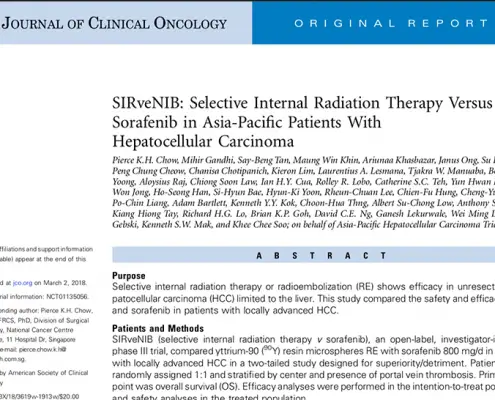
What Is Hepatocellular Carcinoma?
Table of Contents
Hepatocellular Carcinoma (HCC) is the most common type of primary liver cancer. HCC is also the 5th most common cancer globally, the 3rd most common cause of cancer-related deaths, and is estimated to occur at a global rate of 1 million new cases annually. Hepatocellular carcinoma occurs typically in patients suffering from chronic liver diseases, such as cirrhosis caused by hepatitis B or hepatitis C infection.
It is a cancer particularly endemic to East Asia, with rates considerably higher than those in Western societies.
Hepatocellular Carcinoma is often slow to exhibit clinical symptoms. As such, many patients find they only appear at an advanced stage of the disease. Hence, although surgical resection with curative intent is the ideal goal, it is possible in only a small percentage of patients, approximately 10-15%.
Most patients with primary HCC are diagnosed at an intermediate to an advanced stage of the disease, for which there are no standard therapies available.
.
What Is Yttrium-90 Selective Internal Radiation Therapy (SIRT)?
Selective Internal Radiation Therapy (SIRT) is a form of Targeted Brachytherapy / Radiation Therapy developed to treat liver cancers. Sometimes referred to as radioembolisation, it aims to target and destroy liver tumours.
How Does Yttrium-90 Selective Internal Radiation Therapy (SIRT) Work as a Liver Cancer Therapy?
The principle of brachytherapy involves delivering tumoricidal doses of radiation to the cancer but limiting quantities to normal adjacent tissue. Radiation is tumoricidal if sufficient energy can be delivered to the target cells.
Targeted Internal Radiation Therapy offers super-selective therapy of tumours while effectively sparing normal liver parenchyma.
Simply put, small radioactive beads are targeted at a tumour through the patient’s blood vessels in the liver. These radioactive beads give off radiation over a limited, short distance, leaving the surrounding tissue unharmed.
The therapeutic basis of Microsphere Therapy is based on known vascular supply changes occurring in Hepatocarcinogenesis. Typically, liver parenchyma (functional tissue) is supplied mainly via the portal vein with a small supply from the hepatic artery.
In Hepatocellular Carcinoma and some Dysplastic nodules, this is reversed. Hepatic Arterial supply accounts for the majority of vascular supply (approximately 80%), and blood flow is preferentially distributed to the tumour relative to normal liver parenchyma. Meaning that a patient suffering from HCC has a majority of their blood flow being supplied to the tumour as opposed to the functional tissue of the liver.
Yttrium-90 microspheres, when given, will preferentially flow to the tumour because of the increased Arterial Vascular supply. The diameter of SIR-Spheres enables them to become implanted in the tumour microvasculature. However, they are too large to pass through the end arterioles into the hepatic sinusoids, which have a restrictive diameter of 8-10 micron.
This preferential stasis of microspheres within the tumour bed coupled with the limited range and high-energy emission of the Yttrium-90 allows a high tumour radiation dosage with little exposure to surrounding liver parenchyma in theory.
What Are the Expected Outcomes of SIRT?
SIRT is generally a safe and effective liver cancer therapy, with no immediate side effects expected.
Outcomes depend on the team’s experience and are generally better in tertiary institutions with multidisciplinary expertise.
The reported survival benefits are similar to that of oral Sorafenib treatment, which has been considered the standard of care for patients with advanced Unresectable Hepatocellular Carcinomas – where other treatment options have proven ineffective.
However, there are better quality of life scores reported, lower side effects and improved progression, free survival and time to tumour progression.
What Are the Potential Side Effects of Yttrium-90 Selective Internal Radiation Therapy (SIRT)?
Therapy-related complications of SIRT have been shown to run at 2-10%, with outcomes related to the management team’s skill and experience. In approximately one-third of patients, SIRT administration causes immediate short-term abdominal pain, requiring narcotic analgesia, and is typically self-limiting.
Post-SIRT in liver cancer therapy finds lethargy and nausea are common symptoms and can last up to two weeks and may require medication. Most patients develop a mild to moderate fever that may last for several days following SIRT administration. This fever does not usually require treatment.
The most common potential severe complications of SIRT result from either:
- Inadvertent administration of SIR-Spheres into the gastrointestinal tract resulting in gastritis/duodenitis
- Radiation-induced liver disease resulting from a radiation overdose to the normal liver parenchyma
The incidence of gastritis/duodenitis can be reduced by meticulous attention to the administration procedure to ensure a minimal chance of SIR-Spheres entering the numerous small arteries supplying the gastrointestinal tract.
Radiation-induced liver disease is largely, but not entirely, preventable by using appropriate SIRT doses and making allowances for dose reduction when there is an increased risk of causing radiation damage. Such cases include patients with pre-existing liver damage, poor liver reserve or small volume tumour mass in the liver.
The reported incidence of gastritis/duodenitis is less than 10%, while the reported rate of radiation-induced liver disease is less than 1%.
The incidence of Radiation Pneumonitis (inflammation of the lungs due to radiation) is expected to be low where appropriate pre-therapy workup and dose reductions are followed. The risk of radiation pneumonitis nevertheless exists and has been reported.
Who Will Benefit From Yttrium-90 Selective Internal Radiation Therapy (SIRT)?
When considering SIRT in the treatment of liver cancers, patients with intermediate to advanced stage Hepatocellular Carcinomas who are not suitable for curative treatment are most likely to benefit.
Additionally, patients suffering from Metastatic Colorectal Carcinomas with metastases to the liver and Metastatic hypervascular liver lesions such as neuroendocrine tumours are suitable for SIRT.
What Are the Initial Investigations Needed for Patients Considering Yttrium-90 Selective Internal Radiation Therapy (SIRT)?
Your doctor and medical team will need to perform some initial investigations to determine if you are suited for SIRT; these include:
Imaging Studies
- Tri or quadraphonic contrast CT and/or Gadolinium-enhanced MRI of the liver for assessment of tumoral and non-tumoral volume, portal vein patency and extent of extrahepatic disease.
These imaging studies should be obtained within the last 1 month of the intended date of SIRT in liver cancer therapy.
Blood Investigations
- Full Blood Count (FBC)
- Liver Function Test (LFT)
- Renal Function Test
- Cancer Markers (AFP, CEA, Ca 19-9, PIVKA)
- Lactate Dehydrogenase (LDH)
Functional Status
- ECOG Status Score
- Child-Pugh Score
What to Expect During Yttrium-90 Selective Internal Radiation Therapy (SIRT)?
SIRT involves two separate procedures, they are:
- An initial assessment procedure to determine suitability for the actual treatment. Approximately 15% of patients will not be suitable for SIRT after the assessment. Only after passing the assessment study will the treatment be scheduled
- The therapy procedure, which will be scheduled upon successful completion of the initial assessment. The therapy procedure will typically be scheduled on a separate day from the initial assessment procedure.
The interventional radiological procedure for both the assessment and SIRT is similar. The main difference is the type of radioactive nuclide used. The assessment uses a non-toxic diagnostic radionuclide, while SIRT utilises a cytotoxic radionuclide.
On the Day of Yttrium-90 Selective Internal Radiation Therapy (SIRT):
You will be warded on the day of the procedure. Blood investigations will be taken (if not done beforehand), and you will be required to avoid food for 6 hours before the procedure.
Blood transfusions may be arranged as needed. For example, in platelet transfusion during angiography, if the platelet count is <100,000/UL or PT/PTT is elevated, FFP may need to be arranged. You will be given antibiotic prophylaxis as per angiography protocol.
If you are Diabetic and on Metformin, the drug needs to be stopped for two days before and after the procedure.
During Yttrium-90 Selective Internal Radiation Therapy (SIRT):
The Interventional Radiological procedure will be performed in an interventional suite, which is a minor operating theatre. You will be awake during the procedure but may request sedation.
The length of the procedure can vary from 1 – 3 hours, depending on the complexity of the case, and involves a puncture of an artery to allow a catheter insertion into the hepatic artery. Once the catheter is in position, the radionuclide will be injected.
After Yttrium-90 Selective Internal Radiation Therapy (SIRT):
Following the Interventional Radiological procedure, you will be sent to the Nuclear Medicine department to undergo an imaging study.
If there are no complications, you should be discharged from the hospital the day after the Angiogram procedure.
An appointment for the follow-up will be confirmed or arranged for you before discharge.
Yttrium-90 Selective Internal Radiation Therapy (SIRT): Your Questions Answered
What Is Theranostics?
What to Expect: Yttrium-90 Selective Internal Radiation Therapy (SIRT)
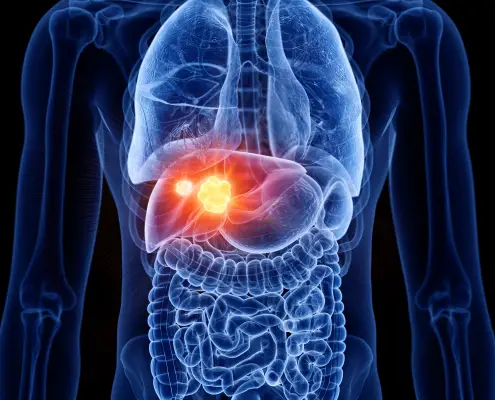
Liver Cancer: Causes, Symptoms, Diagnosis, Treatment
 https://theranostics.sg/wp-content/uploads/2020/05/SIRT-Dosimetry-Technique.jpg
546
750
Dr Andrew Tan
https://theranostics.sg/wp-content/uploads/2020/01/Theranostics_logo-1-300x78.png
Dr Andrew Tan2020-03-30 04:24:462022-05-10 07:53:16SIRT Dosimetry Technique
https://theranostics.sg/wp-content/uploads/2020/05/SIRT-Dosimetry-Technique.jpg
546
750
Dr Andrew Tan
https://theranostics.sg/wp-content/uploads/2020/01/Theranostics_logo-1-300x78.png
Dr Andrew Tan2020-03-30 04:24:462022-05-10 07:53:16SIRT Dosimetry Technique https://theranostics.sg/wp-content/uploads/2020/03/SirFlox-mage.png
1660
1210
Dr Andrew Tan
https://theranostics.sg/wp-content/uploads/2020/01/Theranostics_logo-1-300x78.png
Dr Andrew Tan2020-03-30 04:24:252022-05-10 07:53:37SIRFLOX study evaluating use of SIRT in metastatic colorectal carcinoma
https://theranostics.sg/wp-content/uploads/2020/03/SirFlox-mage.png
1660
1210
Dr Andrew Tan
https://theranostics.sg/wp-content/uploads/2020/01/Theranostics_logo-1-300x78.png
Dr Andrew Tan2020-03-30 04:24:252022-05-10 07:53:37SIRFLOX study evaluating use of SIRT in metastatic colorectal carcinomaWhat are the expected outcomes of SIRT?
SIRT is a generally safe and effective therapy, with no immediate side effects expected.
Outcomes are dependant on the experience of the team, and is generally better in tertiary institutions with multidisciplinary expertise.
The reported survival benefits are similar to that of oral Sorafinib treatment, which has been considered the standard of care for patients with advanced Unresectable Hepatocellular Carcinomas. But there are better quality of life scores reported, lower side effects and improved progression, free survival and time to tumour progression.
What are the potential side effects?
Therapy-related complications have been shown to run at 2-10%, with outcomes related to the skill and experience of the management team.
In approximately one-third of patients, administration of SIRT causes immediate short-term abdominal pain, requiring narcotic analgesia, and is typically self-limiting.
Post-SIRT lethargy and nausea are common symptoms and can last up to two weeks and may require medication.
Most patients develop a mild to moderate fever that may last for several days following SIRT administration. This fever does not usually require treatment.
The most common potential serious complications result from either
- inadvertent administration of SIR-Spheres into the gastrointestinal tract resulting in gastritis/duodenitis or
- radiation-induced liver disease resulting from a radiation overdose to the normal liver parenchyma
The incidence of gastritis/duodenitis can be reduced by meticulous attention to the administration procedure so as to ensure that there is a minimal chance of SIR-Spheres entering the numerous small arteries supplying the gastrointestinal tract.
Radiation-induced liver disease is largely, but not totally, preventable by using appropriate SIRT doses and making allowances for dose reduction when there is increased risk of causing radiation damage such as in pre-existing liver damage, poor liver reserve or small volume tumour mass in the liver. The reported incidence of gastritis/duodenitis is <10%, while the reported rate of radiation induced liver disease is < 1%.
The incidence of Radiation Pneumonitis (inflammation of the lungs due to radiation) is expected to be low where appropriate pre-therapy workup and dose reductions are followed. The risk of radiation pneumonitis nevertheless exists and has been reported.
Who will benefit from SIRT?
- Patients with intermediate to advanced stage Hepatocellular Carcinomas who are not suitable for curative treatment
- Metastatic Colorectal Carcinomas with metastases to the liver
- Metastatic hypervascular liver lesions such as neuroendocrine tumours
What are the initial investigations needed?
Imaging Studies
- Tri or quadraphsic contrast CT and/or Gadolinium-enhanced MRI of liver for assessment of tumoral and non-tumoral volume, portal vein patency and and extent of extrahepatic disease. (These should be obtained within the last 1 month).
Blood Investigations
- Full Blood Count (FBC)
- Liver Function Test (LFT)
- Renal Function Test
- Cancer Markers (AFP, CEA, Ca 19-9, PIVKA)
- Lactate Dehydrogenase (LDH)
Functional Status
- ECOG Status Score
- Child-Pugh Score
What to expect during treatment?
SIRT involves 2 separate procedures:
- Initial assessment procedure to determine suitability for actual treatment. Approximately 15% of patients will not be suitable for SIRT after the assessment. Only after passing the assessment study will the actual treatment be scheduled
- Therapy procedure, upon successful completion of assessment. This will typically be scheduled on a separate day.
The interventional radiological procedure for both the assessment and SIRT is similar. The main difference is the type of radioactive nuclide used. The assessment uses a non-toxic diagnostic radionuclide, while SIRT utilises a cytotoxic radionuclide.
You will be warded on the day of the procedure. Blood investigations will be taken (if not done), and you will be required to avoid food for 6 hours prior to the procedure.
Blood transfusions may be arranged as needed (e.g. platelet transfusion during angiography if platelet count is <100,000/UL, If PT/PTT is elevated FFP may need to be arranged), and you will be given antibiotic prophylaxis as per angiography protocol.
If you are Diabetic and on Metformin, the drug needs to be stopped for 2 days before and after the procedure.
The Interventional Radiological procedure will be performed in an interventional suite, which is a minor operating theatre. You will be awake during the procedure, but can ask for sedation.
The length of the procedure can vary from 1 – 3 hours, depending on the complexity of the case, and involves a puncture of an artery to allow a catheter insertion into the hepatic artery. Once the catheter is in position, the radionuclide will be injected.
Following the Interventional Radiological procedure, you will be sent to the Nuclear Medicine department to undergo an imaging study.
If there are no complications, you should be discharged from hospital the day after the Angiogram procedure.
An appointment for the follow-up will be confirmed or arranged for you prior to discharge.